Symbicort Davis Pdf
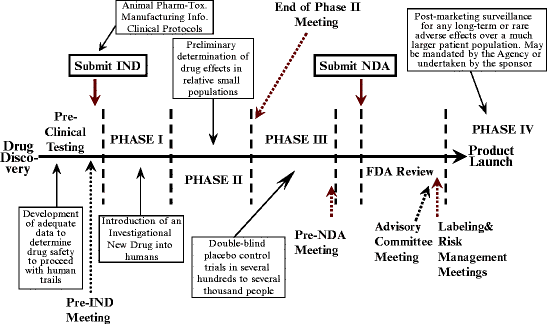
Application Name: Development And Approval Of Inhaled Respiratory Drugs A Us
File Type = .Exe
Credit To @ Approval of Inhaled Respiratory Drugs ...
PDF Download
Open new tab

Application Name: Full Text Comparative Effectiveness Of Budesonide Formoterol
File Type = .Exe
Credit To @ dovepress.com
PDF Download
Open new tab
Application Name: Https Www Asthmaeducators Org Resources Documents New 207 27 2017 20pharmacology 20course Pdf
File Type = .Exe
Credit To @
PDF Download
Open new tab

Application Name: Mosby S Drug Reference For Health Professions 4e Pdf Tahir99
File Type = .Exe
Credit To @ scribd.com
PDF Download
Open new tab
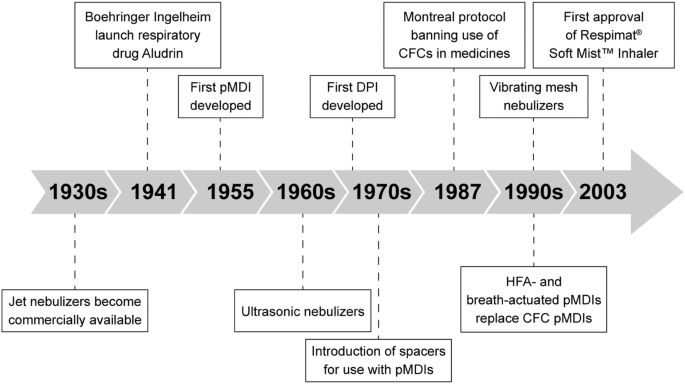
Application Name: The Respimat Soft Mist Inhaler Implications Of Drug Delivery
File Type = .Exe
Credit To @ link.springer.com
PDF Download
Open new tab

Application Name: Tuftsbydrug 0 1 Pharmaceutical Drug Food And Drug Administration
File Type = .Exe
Credit To @ Food And Drug Administration
PDF Download
Open new tab
Capitals indicate life threatening underlines indicate most frequent.
Symbicort davis pdf. Davis company continued pdf page 2 2 assess patients changing from systemic corticosteroids to inhalation corticoste roidsforsigns of adrenal insufciencyanorexianauseaweaknessfatiguehy potension hypoglycemia during initial therapy and periods of stress. 2 dosage and administration. 1 formoterol for mo te role foradilperforomist. Treatment of asthma in patients 6 years of age and older.
1 1 xxxxxx xx 2 3 symbicort 8045 4 budesonide 80 mcg and formoterol fumarate 5 dihydrate 45 mcg inhalation aerosol 6 symbicort 16045 7 budesonide 160 mcg and formoterol fumarate 8 dihydrate 45 mcg inhalation aerosol 9 10 37 mcg formoterol as the free base equivalent to 45 11 mcg formoterol fumarate dihydrate 12 13 for oral inhalation only. 2010 symbicort formoterol budesonide inhaled 343429 drugs drugs formoterolbudesonide inhaled 2002 1413366 overview procedures. If not adequately controlled after 1 to 2 weeks consider an increase to 2 actuations of symbicort 16045 160 mcg budesonide and 45 mcg formoterol per actuation. Pdf page 1 canadian drug name.
Davis and unbound medicine covers 5000 trade name and generic drugs. 11 maintenance treatment of airflow obstruction and reducing exacerbations in patients. Initially 2 actuations of symbicort 8045 80 mcg budesonide and 45 mcg formoterol per actuation inhaled twice daily in the morning and evening approximately 12 hours apart. Symbicort should be administered as 2 inhalations twice daily morning and evening.
21 administration information. Symbicort 16045 is the only strength indicated for the treatment of airflow obstruction in copd. 2 adrenergic agonist indicated for. To view the entire topic please sign in or purchase a subscription.
Symbicort is not indicated for the relief of acute bronchospasm. Symbicort 8045 is a topic covered in the daviss drug guide. Important limitations of use. Davis company continued pdf page 2 2 nursingimplications assessment.

Application Name: Pdf Obesity And Overweight Prevalence Among Adolescents With
File Type = .Exe
Credit To @ Overweight Prevalence Among Adolescents ...
PDF Download
Open new tab